Abstract
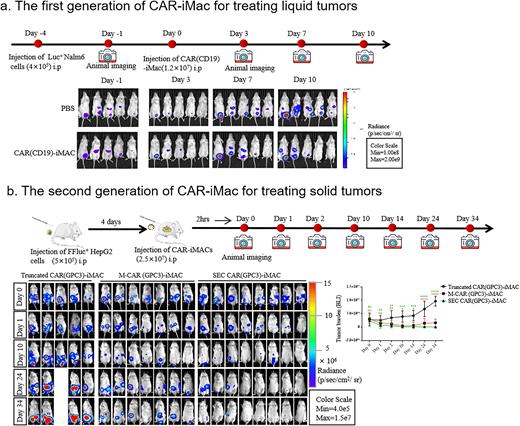
The Chimera antigen receptor (CAR)-T cell therapy has gained great success in the clinic. However, there are still major challenges for its wider applications in a variety of cancer types including lack of effectiveness due to the highly complex tumor microenvironment, and the forbiddingly high cost due to the personalized manufacturing procedures. In order to overcome these hurdles, numerous efforts have been spent focusing on optimizing Chimera antigen receptors, engineering and improving T cell capacity, exploiting features of subsets of T cell or NK cells, or making off-the-shelf universal cells. Here, in the first part, we developed induced pluripotent stem cells (iPSCs)-derived, CAR-expressing macrophage cells (CAR-iMac). CAR expression confers antigen-dependent macrophage functions such as expression and secretion of cytokines, enhanced phagocytosis of tumor cells, and in vivo anticancer cell activity. This technology platform for the first time provides an unlimited source of iPSC-derived engineered CAR-macrophage cells which could be utilized to eliminate cancer cells.
In the second part, we focus on the "second generation” of CAR-iMac and its applications in solid tumors. Therapeutic effects of CAR-T cells on solid tumors remains to be improved. CAR-macrophages have attracted much attention in solid tumor, given their ability to phagocytize tumor cells and their immunomodulatory capacities in the TME. The first generation of engineered CAR-iMac demonstrated that the CAR could stimulate macrophage phagocytosis in a tumor antigen-dependent way. For the second generation of CAR-iMac, after careful screening and rational designs, we genetically engineered iPSC-derived macrophages (iMACs) with Toll-like Receptor intracellular TIR domain-containing CARs against EGFRvIII and GPC3, which yielded an enhanced antitumor effect in two different tumor types . Moreover, CD3ζ-TIR-CAR, the second generation design of TIR-based dual signaling CAR, endowed iMACs with both target engulfment/trogocytosis capacity against antigen-expressing solid tumor cells, and potency of antigen-dependent M1-polarization and M2-like state resistance in an NF-κB dependent manner. Taken together, we established the next generation CAR-iMACs equipped with enhanced phagocytosis and polarization capacity for better antitumor functions in treating liquid and solid tumors.
Disclosures
Zhang:CellOrigin: Current equity holder in private company, Research Funding. Lei:CellOrigin: Research Funding. Zhu:CellOrigin: Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal